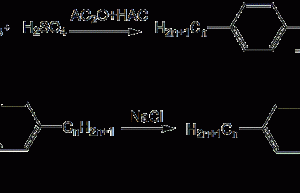
Preparation of bis(4-tert-butylphenyl)iodine chloride_Kain Industrial Additive
Background[1] Since iodonium salt is insoluble in non-polar solvents, the photopolymerization system in which it is used as a photoinitiator must use polar solvents to dissolve the monomers, such as methanol, chloroform, etc. And because its negative ions (AsF6-, SbF6-, etc.) are toxic, its application is limited to a great extent. In response to these…


